AAV (adeno-associated virus) is a type of small DNA virus that has gained significant attention in the field of therapies for cancer or genetic disorder due to its ability to deliver therapeutic genes to specific target cells. AAV capsid tropism refers to the ability of the viral capsid to selectively infect and transduce specific cell types based on the presence of specific surface receptors or other factors on the target cells.
AAV capsid engineering is an important approach in therapies for cancer or genetic disorder research that involves modifying the AAV capsid to improve gene delivery efficiency and specificity. AAV vectors are commonly used in therapies for cancer or genetic disorder due to their ability to efficiently transduce a wide range of cell types without inducing an immune response. However, the AAV capsid can limit the effectiveness of therapies for cancer or genetic disorder in certain cell types or tissues. Capsid engineering allows for the modification of the AAV capsid to enhance transduction efficiency in specific cell types, alter tropism, and improve immune evasion. With ongoing research in AAV capsid engineering, therapies for cancer or genetic disorder holds great promise for the treatment of various genetic disorders and diseases.
Download PackGene’s π-Icosa AAV Capsid Engineering Application note
- Enhance the specificity and efficiency of gene delivery to target cells.
- Minimize the risk of off-target effects and unwanted immune responses.
- Design therapies for cancer or genetic disorder that are more precise and effective in treating specific diseases or conditions.
Never miss the candidate
Combine Directed evolution and rational design
Data you can trust
Consolidated experimental data in animals
Exclusive and confidential
Validated variant sequence will be removed for future projects
Our standard Capsid Engineering service include capsid library design and construction, two rounds of screening and final validation of top variant performance in model animals. Please discuss with our technical support for your screening criteria, animal test and any customized needs.
| π-Icosa standard package* | Animal | Injection | Organ |
| Package 1 | Mouse (Mus musculus) | Intravenous | Any types of Organ except Brain |
| Package 2 | Intravenous | Brain | |
| Package 3 | Cynomolgus monkey (Macaca fascicularis) | Intravenous | Any type of Organ except Brain |
| Package 4 | Intrathecal | Brain |
*Standard packages include two rounds of screening and final validation.
If you need to customize the project, please feel free to contact us.
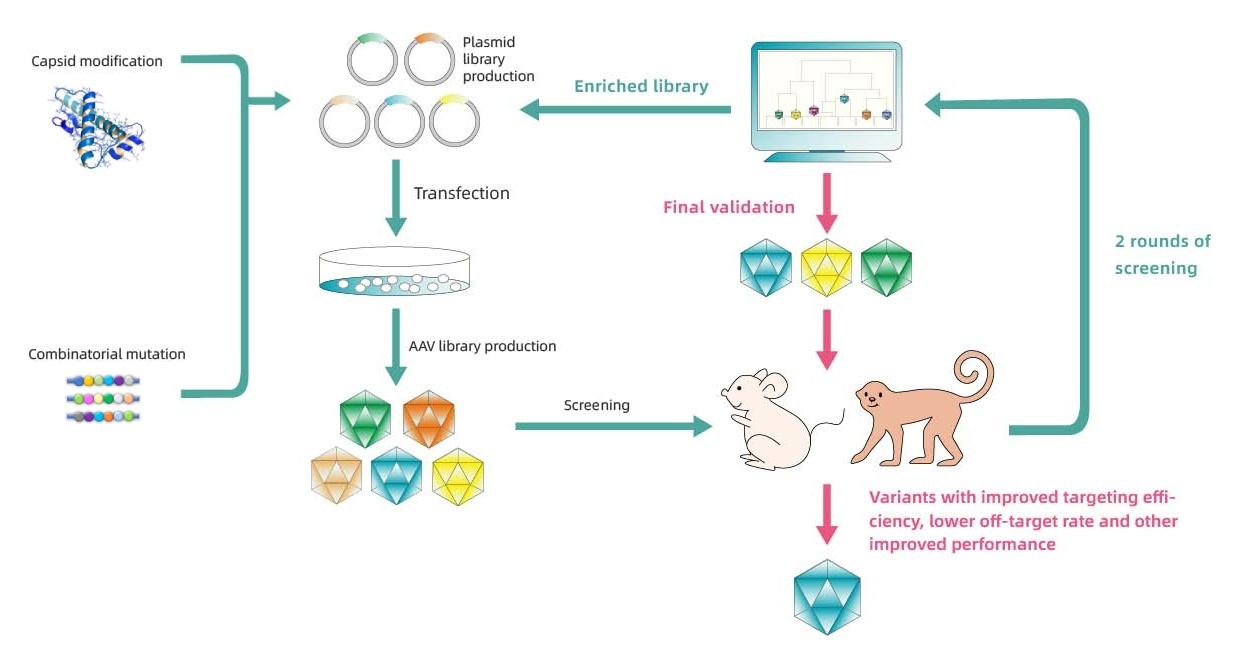
Detailed workflow of Capsid Engineering
PackGene has developed an AAV Capsid engineering platform, π-Icosa system, to engineer and screen AAV capsid variant sequences that have enhanced organ infection, lowered off-targeting, or other features according to customized needs. The process involves three phases of AAV capsid library construction and screening in animals to identify top variants for potential use in GCT. Phase I involves the initial construction of the capsid library, AAV packaging, animal injection, and animal testing, followed by NGS analysis and round 2 library design if necessary. Phase II follow a similar process but started with the modified library based on Phase I screening results to identify the top variants. In Phase III, the top variant plasmids are constructed, AAV packaging is carried out, and animal injection and testing are performed on a larger scale, with potential histology tests available upon request at an additional fee and time. The process aims to identify the most efficient and specific AAV capsids according to your need for GCT development.
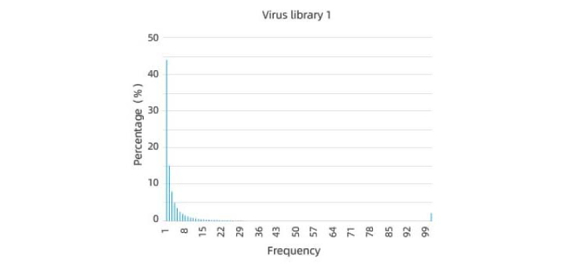
Excellent pre-screening library diversity and uniformity
A high coverage library contains a large number of distinct capsid variants, which increases the likelihood of finding variants with desirable properties. A low coverage library may miss important capsid variants, limiting the potential for capsid engineering. Therefore, it is important to ensure that the library is diverse and contains a large number of distinct variants.
Uniformity is also important because it ensures that each capsid variant is represented equally in the library. Uneven representation can bias the screening process and lead to the selection of suboptimal variants. Therefore, it is important to ensure that the library is evenly distributed and that each variant is represented in similar amounts.
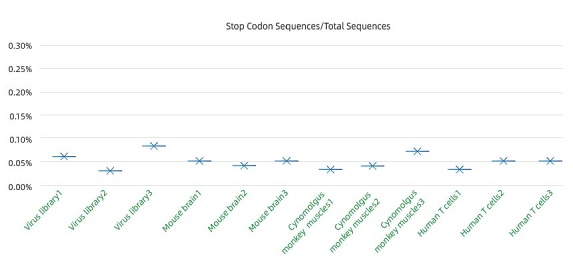
Extremely low mispackaging rate : <0.1%
Percentage of virus packed with early terminated Capsid gene is a key indicator of mispackaging rate. High mispackage rate will lead to mismatch of the capsid serotype with the actual genome it contains, resulting in error in the screening result.
In AAV capsid engineering, the goal is to modify the AAV capsid to improve its efficiency or specificity. One way to achieve this is to generate a library of AAV variants and screen them for desired properties. However, if the AAV vectors produced from this library have a high mispackaging rate, the screening results may be inaccurate and unreliable.
A high mispackaging rate can result in the production of AAV vectors that contain incorrect genetic material. These vectors can then transduce unintended cells or tissues, potentially causing toxicity or reduced efficacy. Additionally, high levels of mispackaging can lead to the formation of replication-competent AAV (rcAAV) particles, which can cause adverse immune reactions and limit the safety of therapies for cancer or genetic disorder.
Therefore, it is important to minimize the mispackaging rate in AAV capsid engineering. This can be achieved by optimizing the vector production process, such as controlling the input DNA amount, using high-quality plasmids, and optimizing the transfection conditions. Additionally, careful screening of the AAV vectors for mispackaging should be performed to ensure that the vectors being tested are representative of the intended capsid library.
To measure mispackaging rate, we measure the rate of AAV population with early terminated capsid protein in the total population. The result indicate we have extremely low mispackaging rate , ensuring the matching of packed AAV capsid gene and the capsid protein during virus packaging, thus lead to reliable screening results.
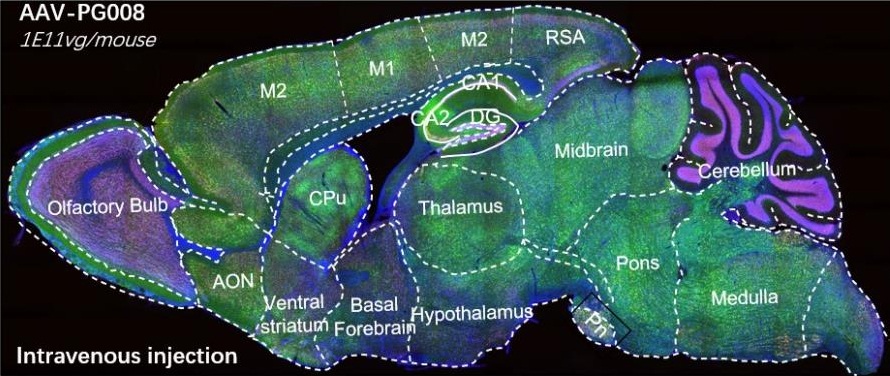
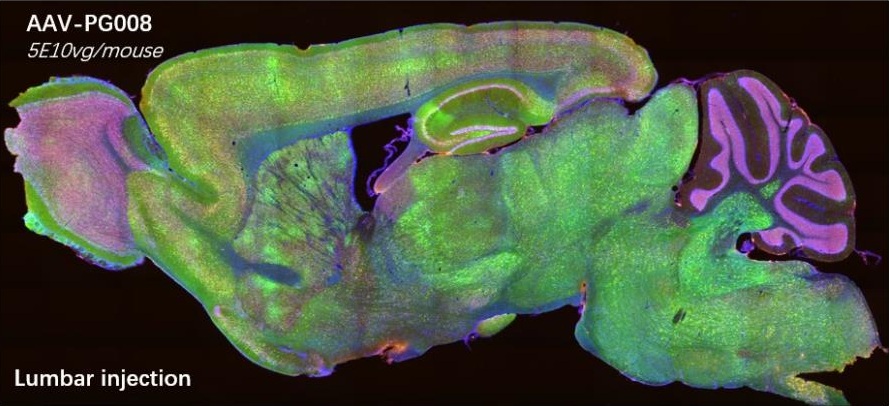
AAV-PG008: a CNS targeting variant engineered via π-Icosa system
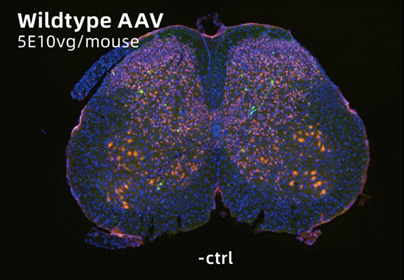
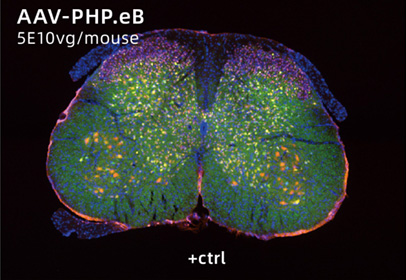
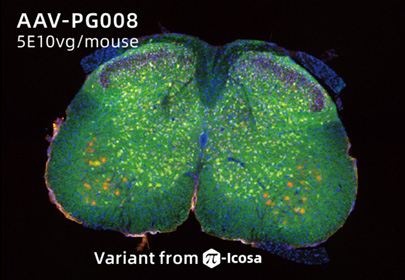
14 days after lumbar injection in mouse. Sample size=5
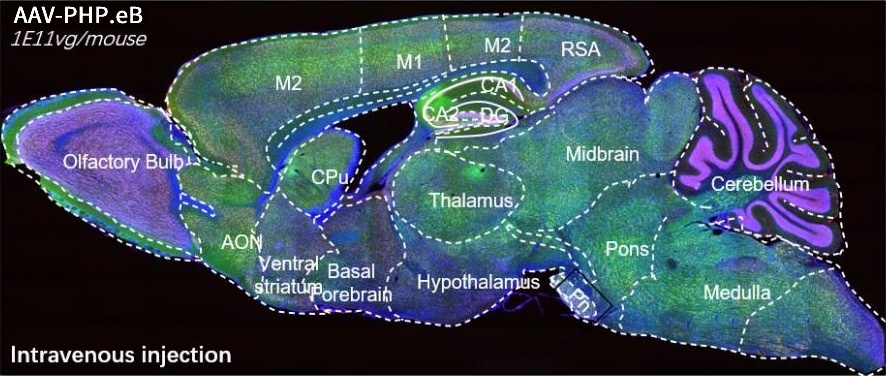
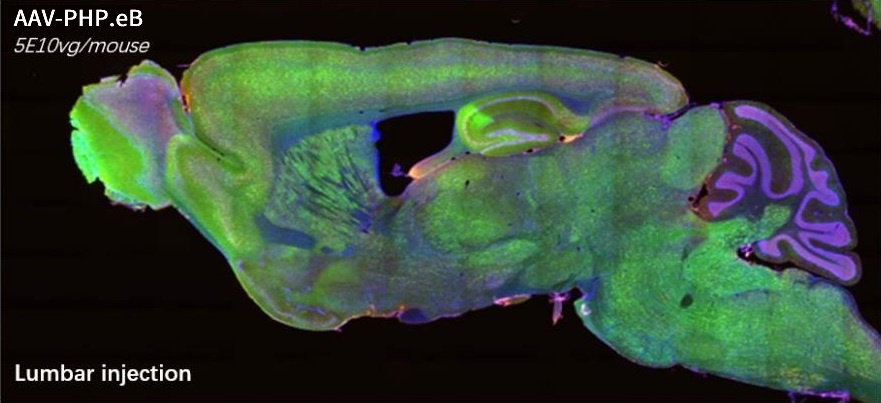
AAV-PHP.eB – a well known serotype that specifically targeted to CNS
AAV-PG008, an variant engineering via π-Icosa
10 days after injection
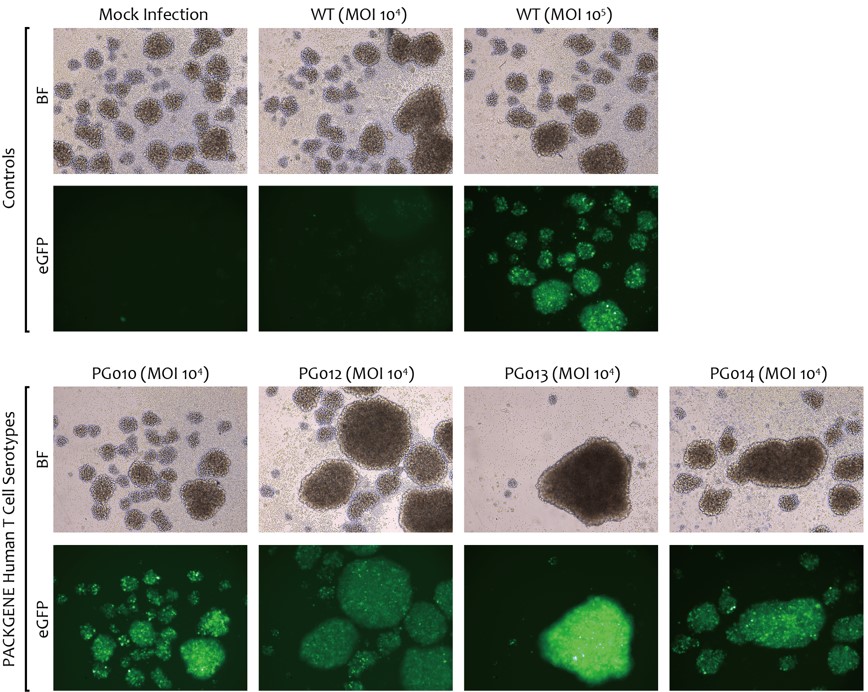
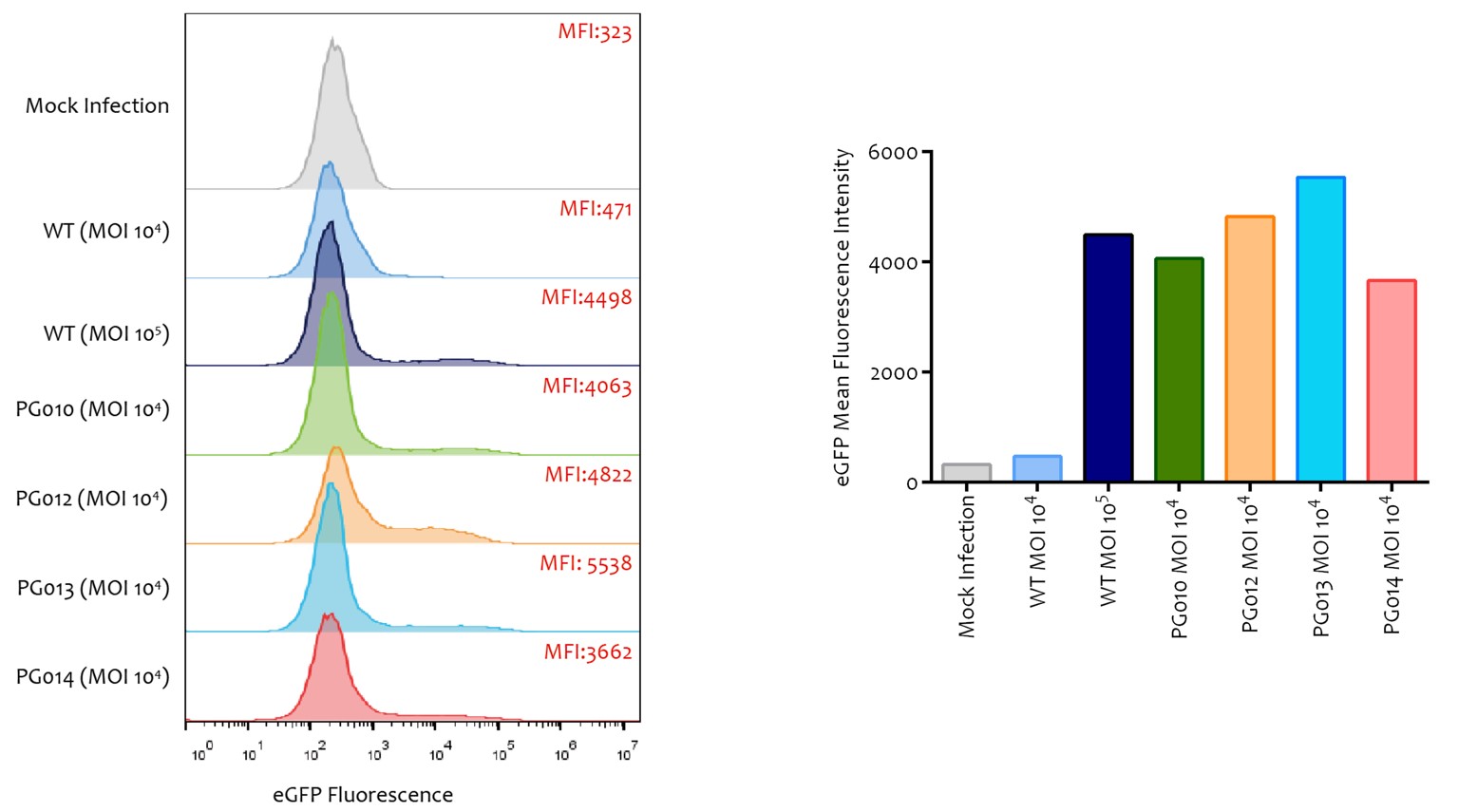
- 4 novel Capsid: PG010, PG012, PG013, PG014
- 10x MOI compared to W

AAV Packaging Services
Ranging from pilot to industrial-scale AAV packaging for both in vitro and in vivo studies.
READ MORE

AAV Analytical Services
We are one of the few vendors that can do ALL in-house.
READ MORE

Off the Shelf AAV Products
We offer a wide catalogue of pre-made AAVs for a multitude of research needs.
READ MORE
